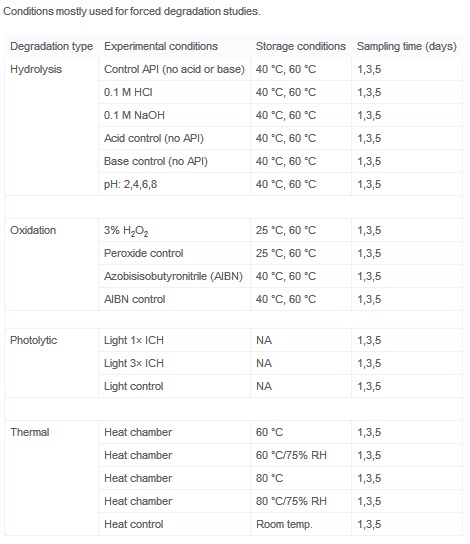
Stability Testing – Doing Everything or Doing the Right Thing? | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
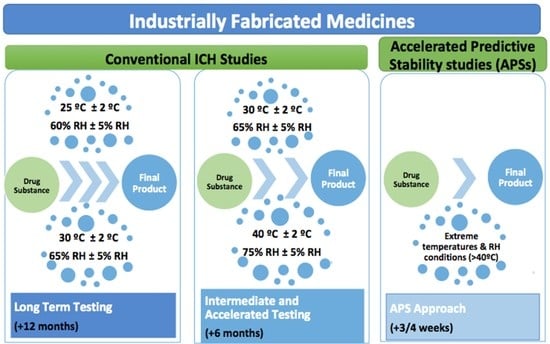
Pharmaceutics | Free Full-Text | Drug Stability: ICH versus Accelerated Predictive Stability Studies
Pharmaceutics | Free Full-Text | Drug Stability: ICH versus Accelerated Predictive Stability Studies
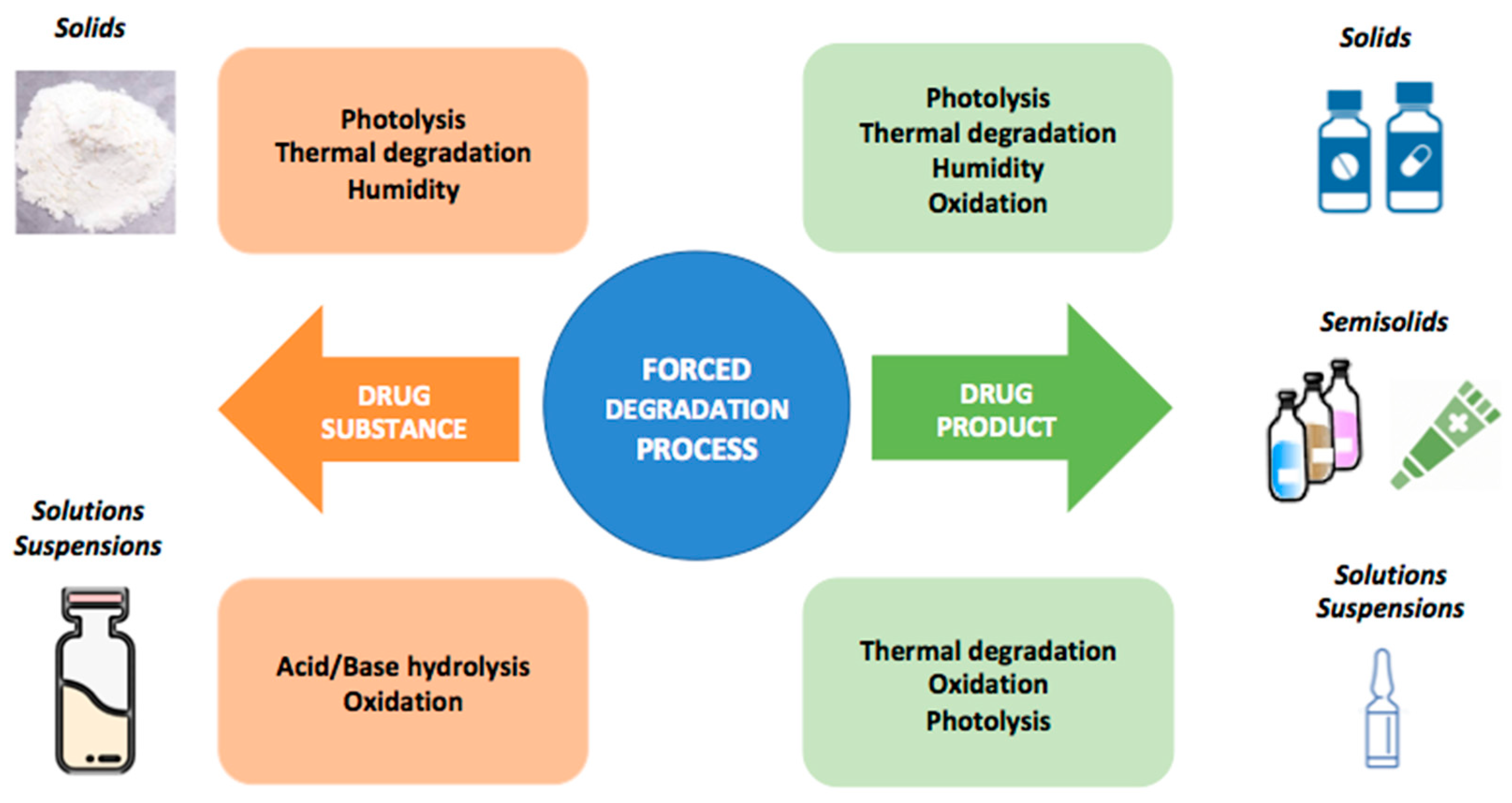
Considerations for Updates to ICH Q1 and Q5C Stability Guidelines: Embracing Current Technology and Risk Assessment Strategies | SpringerLink

Forced degradation studies for Drug Substances and Drug Products- Scientific and Regulatory Considerations | Semantic Scholar
![PDF] Guidance on Conduct of Stress Tests to Determine Inherent Stability of Drugs | Semantic Scholar PDF] Guidance on Conduct of Stress Tests to Determine Inherent Stability of Drugs | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/fce4347f5ddbc12ac1f74d9f7e7ae6db3be2336b/8-Figure2-1.png)
PDF] Guidance on Conduct of Stress Tests to Determine Inherent Stability of Drugs | Semantic Scholar

Product Stability Testing: Developing Methods for New Biologics and Emerging MarketsBioProcess International

Forced Degradation to Develop Stability-indicating Methods | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

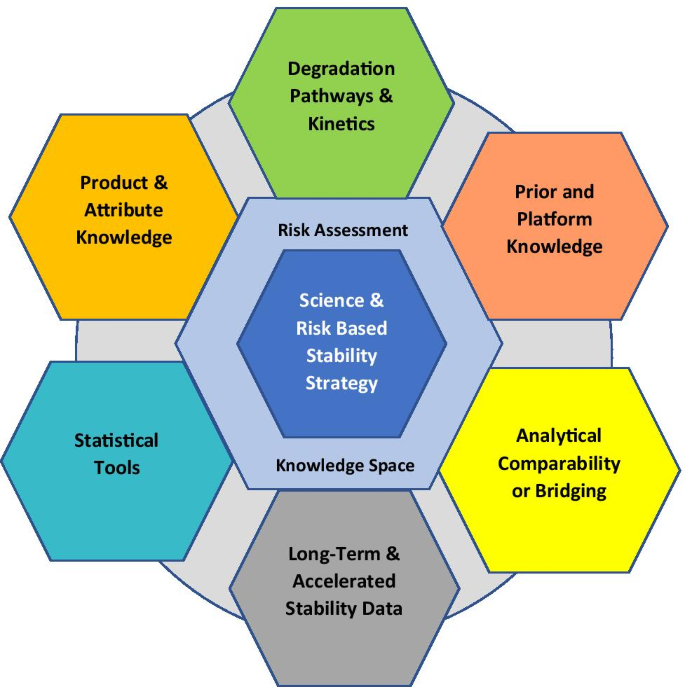
The Degradation Map Process – A Tool for Obtaining a Lean Stability Strategy in Drug Development - Journal of Pharmaceutical Sciences

Degradation conditions for pharmaceutical drugs in forced degradation... | Download Scientific Diagram